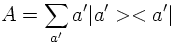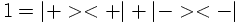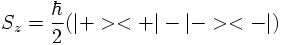The internal degree of freedom called 'spin' had to be introduced in order to interpret the result of the Stern-Gerlach experiment:
A collimated beam of silver atoms is subjected to an inhomogeneous magnetic field with the main component (z-direction) being perpendicular onto the beam direction. If the silver atoms have a magnetic moment (which is essentially the magnetic moment of just one atomic electron) it experiences a force given (to a good approximation) by:
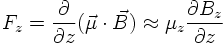
The result of the experiment however is that the beam gets split into two components. The interpretation is that μz and as a consequence Sz can only have two possible values. Numerically, it is found that
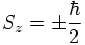
By blocking the |-> component and performing then a Stern-Gerlach experiment in x-direction and blocking the |Sx;->=|-> component and again performing a Stern-Gerlach experiment in z-direction one finds that there are again |Sz;+> and |Sz;-> components in the atomic silver beam.
This leads to the following conclusion: